Abstract
Introduction: Daratumumab (D) is a human monoclonal antibody targeting CD38 that is approved as a monotherapy and in combination with standard of care regimens for patients (pts) with RRMM. Here, we provide an update of CASTOR (NCT02136134), a randomized, multicenter, phase 3 study of DVd vs Vd in pts with RRMM.
Methods: Eligible pts received ≥1 prior line of therapy and were randomly assigned to receive 8 cycles (every 3 weeks) of Vd (V 1.3 mg/m2 subcutaneously on Days 1, 4, 8, and 11; d 20 mg orally or intravenously [IV] on Days 1-2, 4-5, 8-9, and 11-12) with or without D (16 mg/kg IV once weekly in Cycles 1-3, every 3 weeks for Cycles 4-8, and every 4 weeks thereafter until progressive disease [PD]). Pts who progressed on the Vd arm were allowed to cross over and receive D monotherapy. Progression-free survival (PFS) was the primary endpoint. Minimal residual disease (MRD) via next generation sequencing was assessed upon suspected complete response (CR) and at 6 and 12 months after the first dose at 3 sensitivity thresholds (10-4, 10-5, and 10-6) using the clonoSEQTM assay (V.1.3; Adaptive Biotechnologies, Seattle, WA). Cytogenetic risk was determined by next generation sequencing: high-risk pts had t(4;14), t(14;16), and/or del17p abnormalities, and standard-risk pts were confirmed negative for these abnormalities. PFS on subsequent line of therapy (PFS2; defined as time from randomization to PD after next line of subsequent therapy or death) was also examined.
Results : 251 pts received DVd and 247 pts received Vd. The median (range) age was 64.0 (30-88) years. Pts received a median of 2 (1-10) prior therapies; 61% received prior autologous stem cell transplant, 66% received prior bortezomib, 42% received prior lenalidomide, 48% received prior proteasome inhibitor plus immunomodulatory drug, and 28% were refractory to lenalidomide. Median duration of single-agent D in DVd arm after 8 cycles of Vd was 11.9 months.
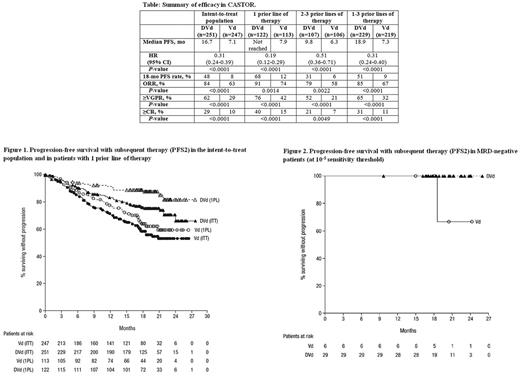
After median follow-up of 19.4 months, PFS was significantly prolonged and the 18-month PFS rate was higher with DVd vs Vd (Table). Significant PFS benefit was observed with DVd regardless of the number of prior lines of therapy received, but the benefit was most pronounced in patients receiving 1 prior line of therapy (Table). The overall response rate and rates of ≥very good partial response (VGPR) and ≥CR were significantly higher with DVd vs Vd (Table).
MRD-negative rates were >3 times higher with DVd vs Vd in the intent-to-treat (ITT) population (19% vs 4% at 10-4, P <0.0001; 12% vs 2% at 10-5, P <0.0001; 5% vs 1% at 10-6, P <0.005) and in pts that received 1 prior line of therapy (24% vs 4% at 10-4, P <0.0001; 14% vs 3% at 10-5, P <0.005; 7% vs 2% at 10-6, P=0.060). At the 10-5 sensitivity threshold, MRD-negativity was associated with prolonged PFS.
PFS2 was significantly improved with DVd vs Vd in the ITT population (hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.40-0.78; P=0.0005; Figure 1). No PFS2 events occurred in MRD-negative pts (at 10-5) treated with DVd (Figure 2). PFS2 was also prolonged in pts who received 1 prior line of therapy (HR, 0.38; 95% CI, 0.21-0.69; P=0.0009; Figure 1) and in pts with ≥VGPR (HR, 0.34; 95% CI, 0.17-0.70; P=0.002). Among high cytogenetic risk pts (DVd, n=44; Vd, n=51), median PFS2 was not reached (NR) in DVd vs 15.9 months in Vd (HR, 0.51; 95% CI, 0.24-1.05; P=0.0647).
Time to next therapy (TTNT) was significantly longer with DVd vs Vd (median NR vs 9.7 months; HR, 0.30; 95% CI, 0.23-0.39; P <0.0001), and in pts who received 1 prior line of therapy (median NR vs 11.1 months; HR, 0.23; 95% CI, 0.15-0.36; P <0.0001). TTNT was also significantly prolonged in high cytogenetic risk pts (median 21.2 vs 9.8 months; HR, 0.38; 95% CI, 0.21-0.71; P=0.0015).
The most common (>10%) grade 3/4 TEAEs were thrombocytopenia (46% vs 33%), anemia (15% vs 16%), and neutropenia (14% vs 5%). A total of 10% of DVd and 9% of Vd pts discontinued treatment due to TEAEs. No new safety signals were reported.
Updated efficacy and safety data will be presented.
Conclusions: DVd continues to significantly prolong PFS and induce deep and durable responses. DVd improved efficacy regardless of prior lines of therapy, with pts who received only 1 prior line of therapy benefitting the most. Durable responses with 11.9 months of single-agent D in the DVd arm translated into longer PFS2 and TTNT. The safety profile of DVd remains consistent with earlier reports after longer follow-up.
Spencer: Janssen: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Hungria: Celgene, Roche, Takeda, Janssen, Amgen: Honoraria. Mateos: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Nooka: Amgen, Novartis, Spectrum, Adaptive tecnologies: Consultancy. Estell: Janssen: Membership on an entity's Board of Directors or advisory committees. Corradini: Sanofi: Honoraria; Roche: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Gilead: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Novartis: Honoraria. Thiyagarajah: Janssen: Employment. Deraedt: Janssen: Employment. Chiu: Janssen: Employment. Schecter: Janssen: Employment. Weisel: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal